Solvents Visualization in Hansen Space
This is an MATLAB visualization program to view solvents in hansen space. Additionally, the program allows to encapsulate “good solvents” within a sphere and shows the solvents in 2d space for better visualization. Another feature of the program is listing all the solvents and corresponding RED values in a tabular format. For further information please take a look at the article and the readme file.
Hansen Program Download.
Publication:
-
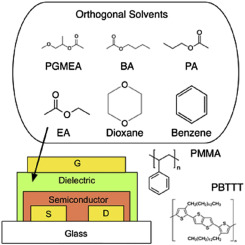
Identifying orthogonal solvents for solution processed organic transistors
Abhinav M. Gaikwad,
Yasser Khan,
Aminy E. Ostfeld,
Shishir Pandya,
Sameer Abraham,
and
Ana Claudia Arias
Organic Electronics,
2016
30,
[Abstract]
[Bibtex]
[PDF]
Solvents visualization program is available in the Downloads section.
Abstract Identification of solvents for dissolving polymer dielectrics and organic semiconductors is necessary for the fabrication of solution-processed organic field effect transistors (OFETs). In addition to solubility and printability of a solvent, orthogonality is particularly important when forming multilayer structure from solutions. Currently, the process of finding orthogonal solvents is empirical, and based on trial-and-error experimental methods. In this paper, we present a methodology for identifying orthogonal solvents for solution-processed organic devices. We study the accuracy of Hildebrand and Hansen solubility theories for building solubility boundaries for organic semiconductor (Poly(2,5-bis(3-hexadecylthiophen-2-yl)thieno[3,2-b]thiophene (PBTTT) and polymer dielectrics (Poly(methyl methacrylate) (PMMA), Polystyrene (PS)). The Hansen solubility sphere for the organic semiconductor and polymer gate dielectrics are analyzed to identify solvents that dissolve {PMMA} and PS, but are orthogonal to PBTTT. Top gate/bottom contact {PBTTT} based {OFETs} are fabricated with {PMMA} gate dielectric processed with solvents that are orthogonal and non-orthogonal to PBTTT. The non-orthogonal solvents swell the semiconductor layer and increase their surface roughness.
@article{gaikwad2016identifying,
title = {Identifying orthogonal solvents for solution processed organic transistors},
author = {Gaikwad, Abhinav M. and Khan, Yasser and Ostfeld, Aminy E. and Pandya, Shishir and Abraham, Sameer and Arias, Ana Claudia},
journal = {Organic Electronics},
volume = {30},
pages = {18 - 29},
year = {2016},
issn = {1566-1199},
url = {http://www.sciencedirect.com/science/article/pii/S1566119915302299},
doi = {10.1016/j.orgel.2015.12.008},
thumbnail = {gaikwad2016identifying.png},
pdf = {gaikwad2016identifying.pdf},
publisher = {},
note = {Solvents visualization program is available in the Downloads section.}
}
Bulk Heterojunction Device Model Simulator Package
This is a simulation package for bulk heterojunction organic photovoltaics from J. Appl. Phys. 113, 154506 (2013). The carrier concentration boundary conditions, mobility, exciton quenching efficiency, optical constants, dielectric constants and recombination rates can be entered into the simulator as experimentally-determined parameters. The program computes generation, recombination rates, and carrier concentration as a function of position in the active layer as well as photocurrent as a function of applied bias. Additionally, the morphology of the active layer can be varied as a function of position in the active layer to simulate graded devices. A readme file can be found inside the package with detailed instructions.
Simulator Package Download.
Publication:
-
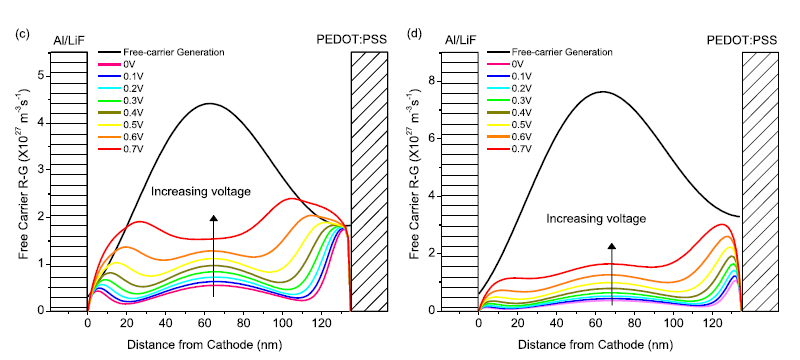
Empirically based device modeling of bulk heterojunction organic photovoltaics
Adrien Pierre,
Shaofeng Lu,
Ian A. Howard,
Antonio Facchetti,
and
Ana Claudia Arias
Journal of Applied Physics,
2013
113,
15.
[Abstract]
[Bibtex]
[PDF]
Bulk heterojunction device model simulator package is available in the Downloads section.
We develop an empirically based optoelectronic model to accurately simulate the photocurrent in organic photovoltaic (OPV) devices with novel materials including bulk heterojunction OPV devices based on a new low band gap dithienothiophene-DPP donor polymer, P(TBT-DPP), blended with PC70BM at various donor-acceptor weight ratios and solvent compositions. Our devices exhibit power conversion efficiencies ranging from 1.8% to 4.7% at AM 1.5G. Electron and hole mobilities are determined using space-charge limited current measurements. Bimolecular recombination coefficients are both analytically calculated using slowest-carrier limited Langevin recombination and measured using an electro-optical pump-probe technique. Exciton quenching efficiencies in the donor and acceptor domains are determined from photoluminescence spectroscopy. In addition, dielectric and optical constants are experimentally determined. The photocurrent and its bias-dependence that we simulate using the optoelectronic model we develop, which takes into account these physically measured parameters, shows less than 7% error with respect to the experimental photocurrent (when both experimentally and semi-analytically determined recombination coefficient is used). Free carrier generation and recombination rates of the photocurrent are modeled as a function of the position in the active layer at various applied biases. These results show that while free carrier generation is maximized in the center of the device, free carrier recombination is most dominant near the electrodes even in high performance devices. Such knowledge of carrier activity is essential for the optimization of the active layer by enhancing light trapping and minimizing recombination. Our simulation program is intended to be freely distributed for use in laboratories fabricating OPV devices.
@article{pierre2013empirically,
author = {Pierre, Adrien and Lu, Shaofeng and Howard, Ian A. and Facchetti, Antonio and Arias, Ana Claudia},
title = {Empirically based device modeling of bulk heterojunction organic photovoltaics},
journal = {Journal of Applied Physics},
year = {2013},
volume = {113},
number = {15},
eid = {154506},
pages = {},
url = {http://scitation.aip.org/content/aip/journal/jap/113/15/10.1063/1.4801662},
doi = {10.1063/1.4801662},
thumbnail = {pierre2013empirically.png},
pdf = {pierre2013empirically.pdf},
publisher = {},
note = {Bulk heterojunction device model simulator package is available in the Downloads section.}
}
Last modified: 2022-04-12
 Identifying orthogonal solvents for solution processed organic transistors Organic Electronics, 2016 30, [Abstract] [Bibtex] [PDF] Solvents visualization program is available in the Downloads section.
Identifying orthogonal solvents for solution processed organic transistors Organic Electronics, 2016 30, [Abstract] [Bibtex] [PDF] Solvents visualization program is available in the Downloads section. Empirically based device modeling of bulk heterojunction organic photovoltaics Journal of Applied Physics, 2013 113, 15. [Abstract] [Bibtex] [PDF] Bulk heterojunction device model simulator package is available in the Downloads section.
Empirically based device modeling of bulk heterojunction organic photovoltaics Journal of Applied Physics, 2013 113, 15. [Abstract] [Bibtex] [PDF] Bulk heterojunction device model simulator package is available in the Downloads section.